ENDOMETRIAL RECEPTIVITY ASSAY (ERA)

Dr. Anannya Chakraborty
Infertility & IVF Expert
Institute of Human Reproduction
Infertility, that is inability to conceive, is not merely a health issue, but it also causes immense social and psychological stress. Even couples undergoing IVF treatment are under extreme anxiety and emotional turmoil, which is beyond any measure. To add to the woe, recurrent IVF failures pose utmost disappointment both for the couple as well as the doctor. The underlying cause can not only be the embryo, but also the endometrium – which is the “nest” where the embryo implants. So, let’s understand the important “E”s in the process of implantation – Embryo, and Endometrium.
Embryo
Around 60% of all miscarriages are caused due to abnormalities in the embryo. Transferring the best quality embryos improves the IVF outcome by many folds. When there is no clinical pregnancy even after transferring at least four good-quality embryos in a minimum of three IVF cycles in a woman less than 40 years, then it is called Recurrent Implantation Failure (RIF).
Endometrium
The endometrium is the tissue that lines the uterine cavity. This is a dynamic layer that undergoes a series of events in response to the hormonal cycle in a female body. The first half of the cycle is marked by follicular growth in the ovary and endometrial thickening under the influence of the hormone, Estrogen. After the egg is released from the ovary, the ruptured follicle turns into Corpus Luteum that secretes the hormone, Progesterone. Estrogen and Progesteroneproduce numerous endometrial proteins by a process called “Gene expression”. If there is no implantation, the endometrial layer sheds off in the form of menstruation.
Importance of endometrail receptivity
According to researchers, the fine-tuning between the status of the endometrial lining and the developmental stage of the embryo is the key to the success of treatment. The embryo is able to implant only during a specific receptive period of time, called the Window Of Implantation (WOI). This window can vary from person to person.
The Endometrial Receptivity Assay (ERA) is an advanced genetic diagnostic tool to assess endometrial receptivity and to determine each woman’s personalized embryo transfer (pET) timing, from a molecular point of view. The hormones, Estrogen, and Progesterone produce endometrial proteins by gene modulation and expression. The ERA test analyzes the expression of 248 such genes related to endometrial receptivity. For some women, the period of receptive endometrium may be a few days earlier or later than usual, hence the date of embryo transfer may need to be adjusted accordingly.
Before the advent of this technology, the only way to assess the endometrium was through ultrasound, wherein the endometrial thickness could be measured. However, it was seen that even with the transfer of high-quality embryos to a good thickness endometrium, there was a failure to achieve pregnancy. ERA biopsies have helped in achieving that extra edge in such cases by analyzing the molecular status of the endometrium in conjugation with the endometrial thickness. ERA also helps to determine whether the culprit behind Recurrent Implantation Failure or Recurrent Pregnancy Loss is the inappropriate endometrium.
Who should consider ERA?
ERA is not a part of the usual female fertility assessment. However, in cases of Recurrent IVF Failures or Recurrent miscarriages, ERA biopsy may be an option.
How is ERA done?
The process begins one cycle prior to Frozen Embryo Transfer (FET). The woman is started on hormonal supplementation as if being prepared for FET. On the standard day of embryo transfer, a 10-30 seconds endometrial biopsy is done instead of Embryo Transfer. It is done as an outpatient procedure, wherein the endometrial layer is “scratched” with a Pipelle. This may cause mild bleeding or cramping of the lower abdomen. The sample so obtained is then sent to the ERA laboratory for molecular analysis by Next Generation Sequencing (NGS). The biopsy sample is also called ERA Biopsy and the whole process is named ERA testing.
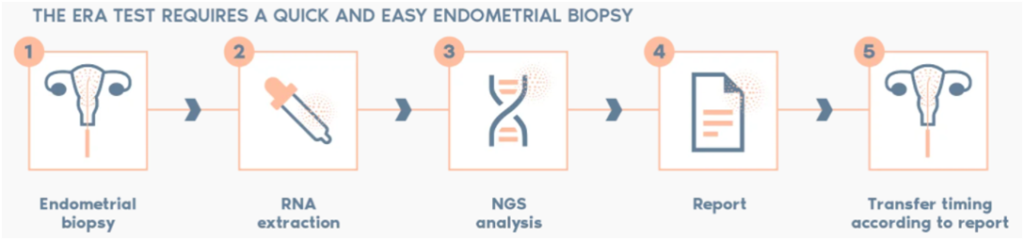
In a natural cycle, a biopsy is done on LH+7 (LH Surge + 7 days) or on P+5 (Start of progesterone + 5 days) in a hormone-stimulated cycle. The report classifies the endometrium as Receptive, Pre- or Post-receptive. The ERA group claims that the results are consistent, i.e. a woman’s WOI doesn’t change with time, although a displaced non-receptive endometrium has been seen in 20-25% of cases. Hence, this test enables us to identify the unique personalized WOI for each patient in the majority of cases.
RCT Conclusions
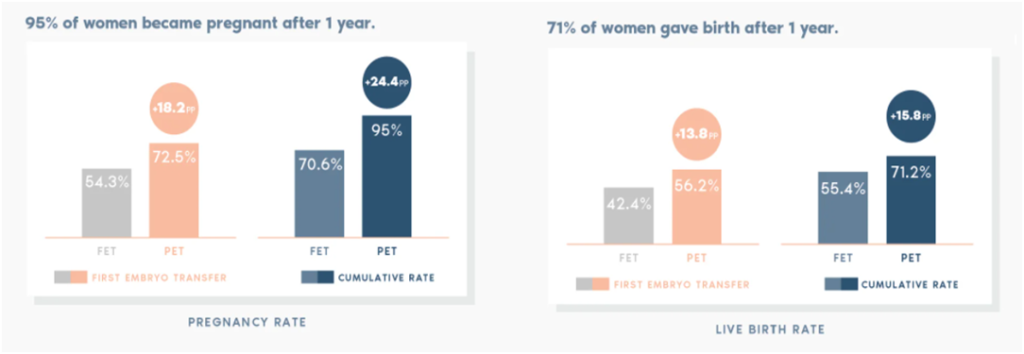
The ERA test has been found to improve the cumulative pregnancy rate by almost 25% and the cumulative live birth rate by about 16% globally.
Cost of ERA
The ERA test costs around Rs 50,000 (INR), which includes the test fee, courier charges, and Pipelle biopsy, hospital charges, and consultant fees.
Future of ERA

Although ERA is a step ahead towards personalized medicine, it is not always a definitive answer to the grey zone of Recurrent Implantation Failure. However, by synchronizing the embryo transfer with the WOI, ERA may show promising results in cases of Recurrent Implantation Failure and give a new ray of hope to infertile couples.


This post is extremely radiant. I extremely like this post. It is outstanding amongst other posts that I’ve read in quite a while. Much obliged for this better than average post. I truly value it!